KBI Biopharma's
SUREtechnology Platform™,
powered by Selexis®
SUREmAb™ for Rapid Development
Introducing our vertically-integrated approach for the streamlined production of therapeutic monoclonal antibodies from cell line development to cGMP manufacturing
Guaranteed minimum titers of 4 g/L at the RCB stage, with tox material available in 5 months from a pool of clones and 6 months from top clones*
Talk to our Experts
Streamlined & Cost-Effective Monoclonal Antibody Integrated Offering
SUREmAbTM accelerates Monoclonal Antibody (mAb) CLD, bringing your project from transfection to Research Cell Bank (RCB) in as little as 9 weeks*, with guaranteed tox material available in 5 months from a pool of clones and 6 months from top clones*, and drug substance release in 11 months*.
By the end of your program, you will have:
- Guaranteed: Minimum titers of 4 g/L
- High-performing, monoclonal SURE CHO-M Cell Line (MCB) expressing your monoclonal antibody
- Qualified analytical methods
- Scalable and high-yield drug substance manufacturing process
- Drug substance / drug product formulation
- cGMP bulk drug substance with Certificate of Analysis
- ICH-compliant drug substance and drug product release and stability
- Development reports to support IND regulatory filing

Specific
Precision-engineered for a broad range of molecules

Unique
Proprietary platform built on patented technologies

Robust
Rapid, reliable, and versatile for producing recombinant proteins

Efficient
Streamlined and timely from early-stage to GMP manufacturing
Built on the SUREtechnology Platform™
Your mAb development program is secured from the start by building its foundation upon the SUREtechnology Platform, a global technology leader in cell line development technologies. With over 20 years of experience, the SUREtechnology Platform guarantees titers of 4 g/L at RCB stage, and deliver titers up to 18 g/L in fed-batch processes. With SUREmAb, we have applied our know-how to develop an optimal workflow for secured speed, enabling your program development and manufacturing for standard IgG1, IgG2, and IgG4 that brings you from transfection to RCB in 9 weeks*, with guaranteed tox material available in 5 months from a pool of clones and 6 months from top clones*, and into drug substance release in 11 months*.
Join a legacy of success with our SUREtechnology Platform™
0
Years of mAb Development Experience
0
Therapeutic mAb Projects
0
Commercialized mAb Therapeutics
Proven Scalability from CLD to PD
Robust CLD that Scales
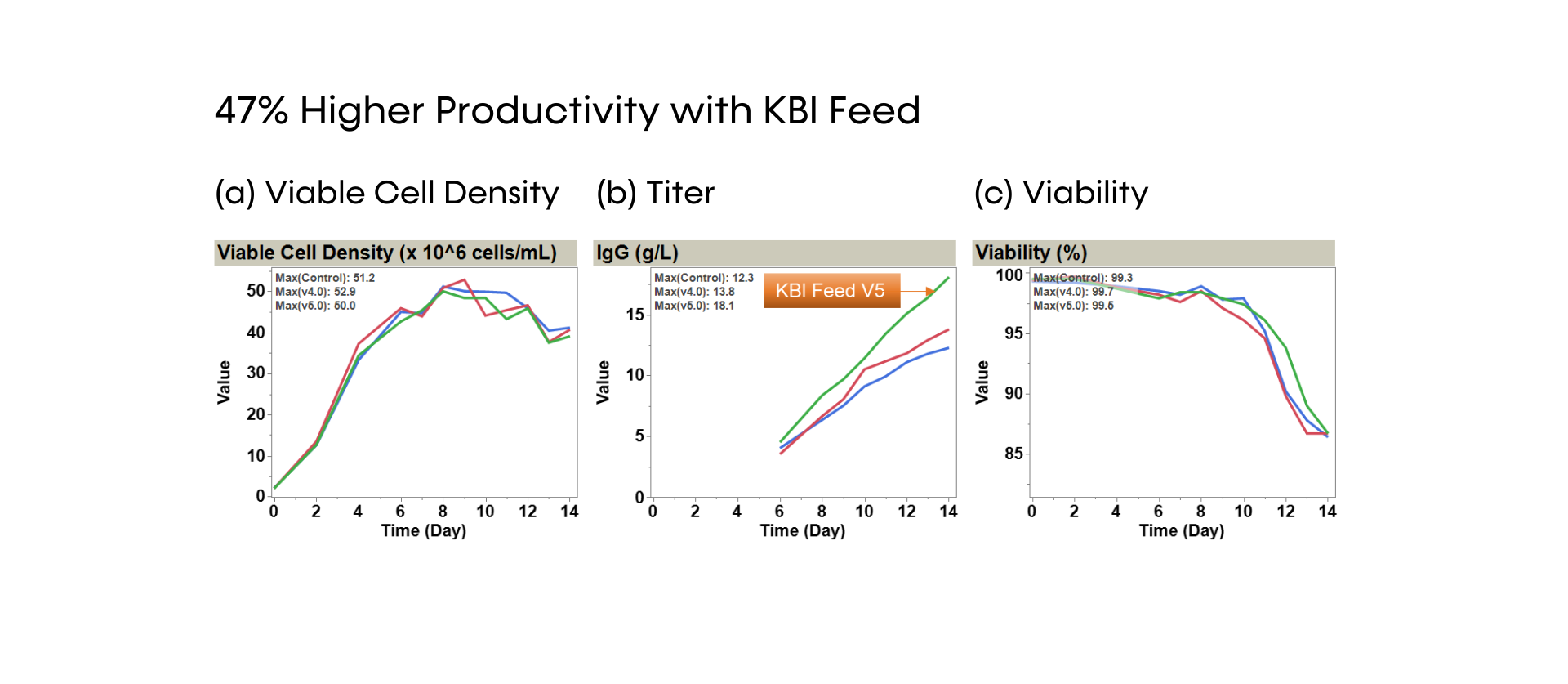
Across scales from 15 mL to 200 L bioreactors, KBI Biopharma has demonstrated that its SUREmAb-generated SURE CHO-M Cell Line is robust in cell density, viability, and productivity profiles.
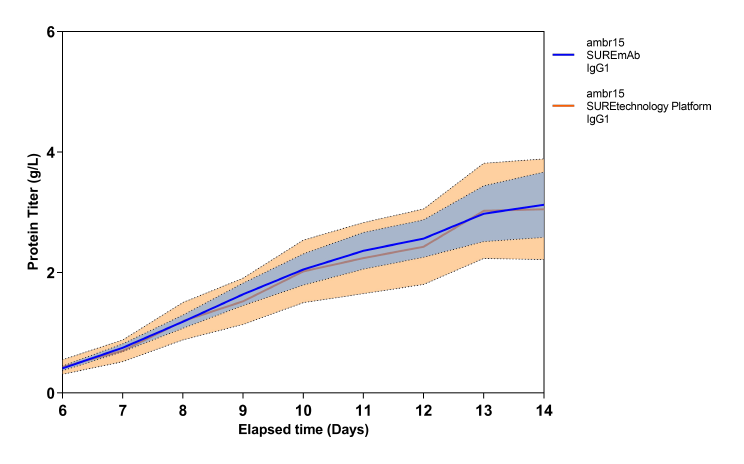
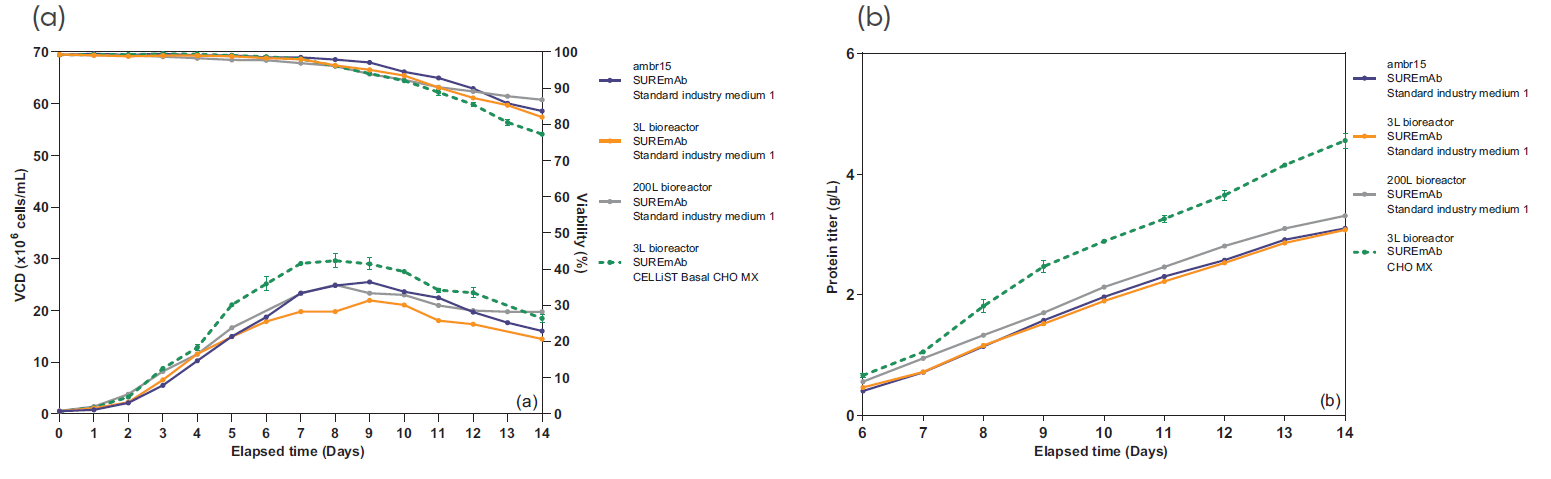
Fig. 2 - SUREmAb scalability. VCD, viability (a) and productivity (b) profiles of one stable RCB expressing an IgG1 in 3 different scales: ambr® 15, 3L and 200L bioreactors. Two chemically defined media were used: standard industry medium 1 (straight lines) and CHO-optimized medium CELLISTTM Basal CHO MX (dotted line).
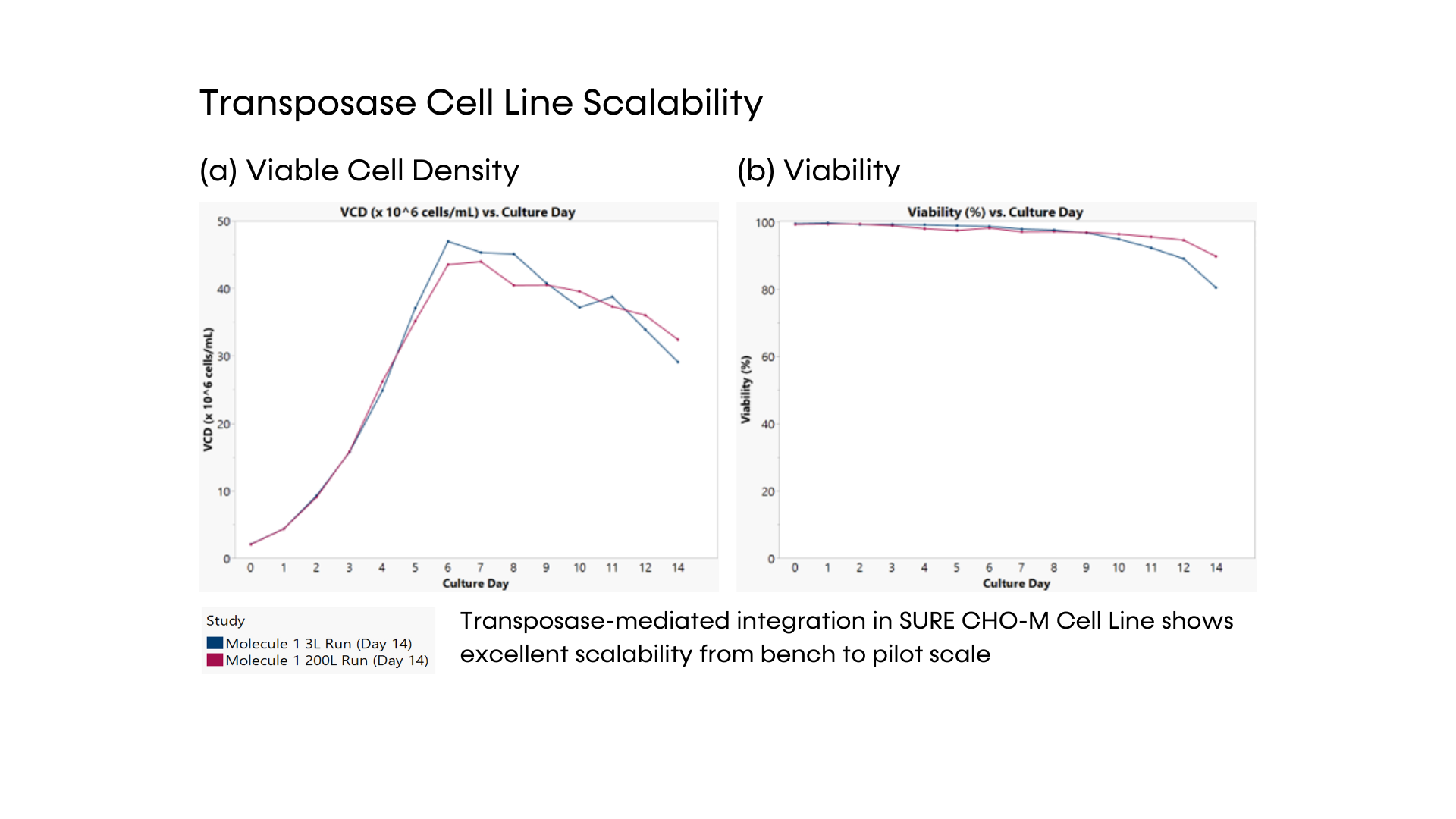
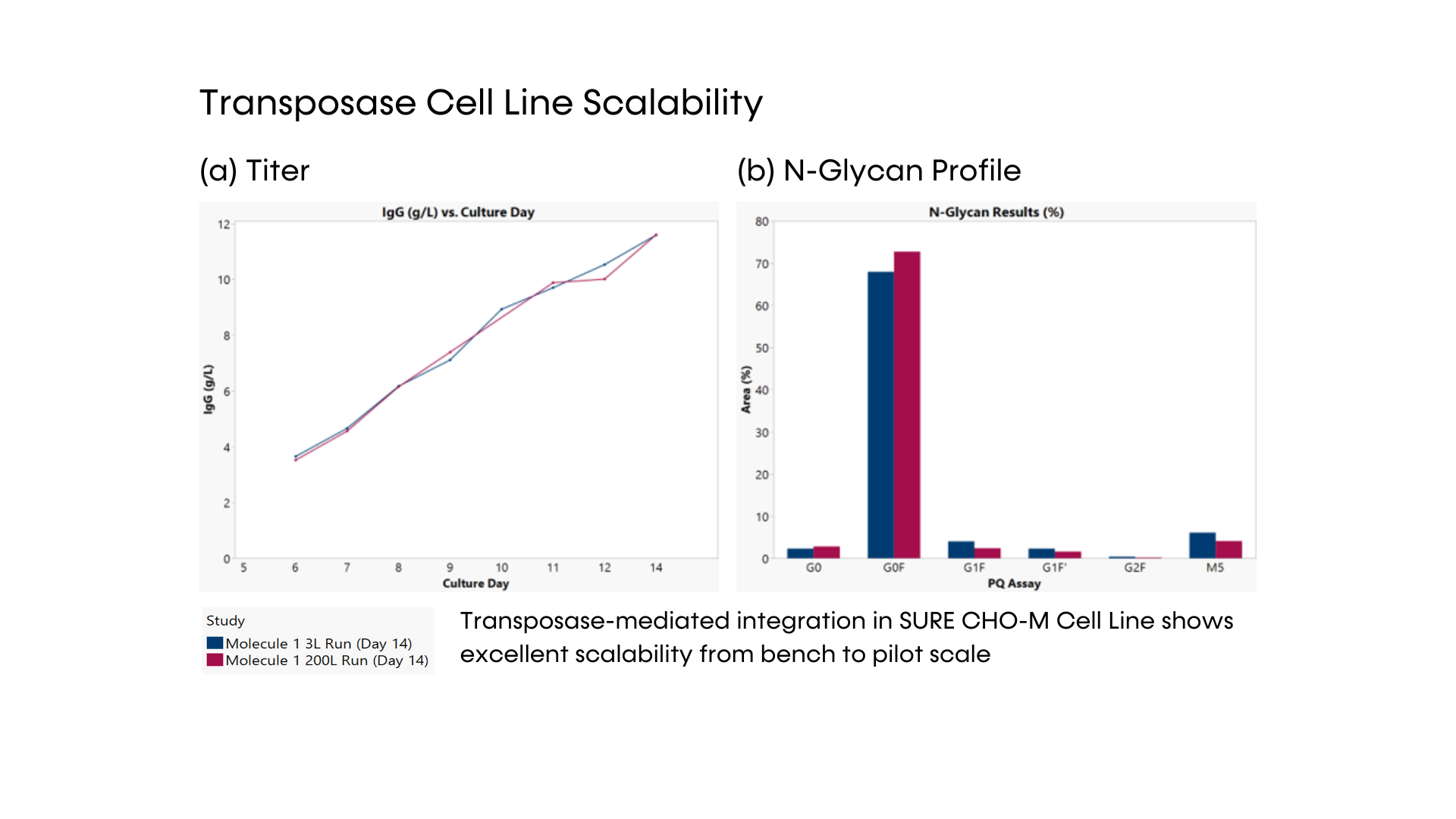
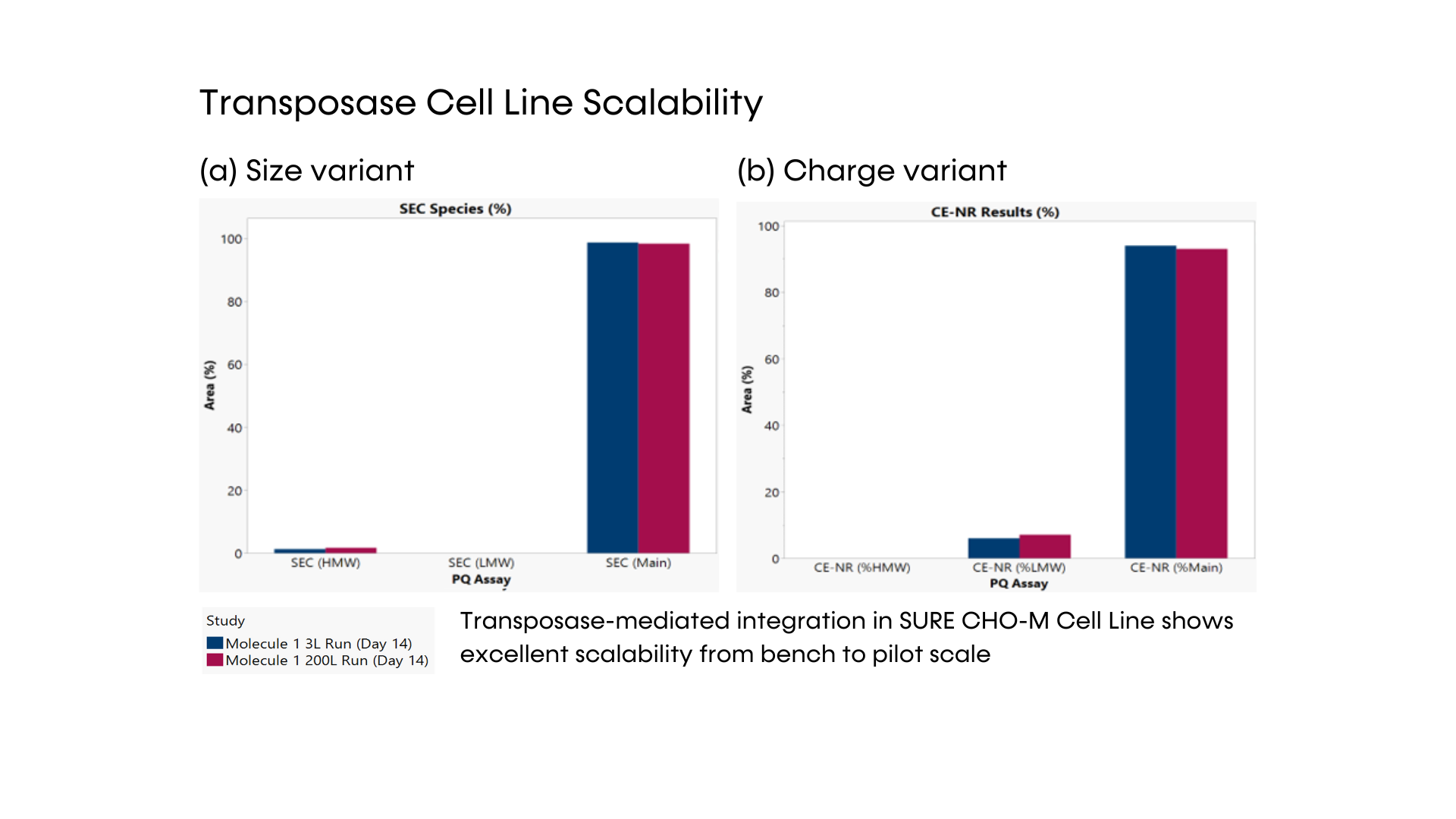
Fig. 3 – Transposase cell line scalability. VCD (a) and viability (b) of one stable transposase cell line in 2 different scales: 3L and 200L bioreactors.
Our Decades of Experience Have Enabled Us to Design an Optimal CLD Workflow for Secure, Speedy Development for Your Program
Streamlined & Robust Science Supports Secure mAb Development
Proprietary Tools
Using our proprietary CLD technology and our SURE CHO-M Cell Line, we optimize your transgene codon sequence, match it with the best signal peptides, and insert it in our proprietary vector backbone containing proprietary epigenetic elements optimized for the best expression and secretion levels.
High-Producing from the Start
Using ClonePix, we are able to screen for high-producer pools from an early stage. Combining cell colony imaging technology with labeling your molecule, only the most promising pools in terms of expression and viability are selected for clone isolation. The robustness of this step provides exceptional security and confidence right from the start.
Monoclonality, Assured
Using Beacon instrumentation, we confirm - with visual proof - that your cell line is monoclonal in origin prior to RCB banking. This offers even more robust confirmation of monoclonality than the traditional statistical calculation from FACS or limited dilution methodologies.
Designed for Scale-up
Throughout the entire development process, we maximize the relevancy of results and accuracy of prediction by using Ambr® 15 bioreactors as early as CLD. In the Process Development (PD) phase, we use Ambr 250 bioreactors before transitioning to 3 L and 200 L scale. The use of bioreactors from the start enables us to monitor cellular growth and nutrients in a non-invasive way, mimicking conditions at scale while eliminating the need for pipetting and the associated risks like contamination.
Single-Use Manufacturing
Risk elimination continues into manufacturing scale, where we employ single-use bioreactors to eliminate cross-contamination while decreasing cost of goods. The segregated HVAC design and personnel, materials, and equipment fit together with the deployment of end-to-end single-use technology for rapid turn-around, further protecting timelines and investments.
Streamlined and Robust Operations
Parallel Activities Accelerate your mAb Program
SUREmAb streamlines monoclonal antibody development, accelerating your program by running activities in parallel. You work with a single Project Manager from start to finish, ensuring seamless tech transfer across functions while staying in continuous communication with subject matter experts to track program progress throughout development.
Beginning Process Development activities with single-cell clones in small-scale bioreactors leads to accelerated process lock and scale-up while Analytical and Formulation activities run in parallel. At the end of your program, you receive a documentation package that supports your regulatory IND filing, providing you with comprehensive and transparent documentation that gets your therapeutic mAb to patients, faster.

Upon reception of your RFP, you will receive a single proposal that covers every step of your SUREmAbTM program. Once the contract is signed, your project starts CLD without delay. We go a step further in streamlining your full-scope experience with the alleviation of license fees for SUREmAb programs that continue into manufacturing with KBI Biopharma.
Development Success is a SURE Thing with SUREmAb
With optimized processes for efficiency and speed, SUREmAb delivers high titers with lower-cost workflows
Ask us about our royalty-free option
Innovation with Alleviation of Royalties
By continuing your project into manufacturing with KBI, we can waive the license fees for GMP clinical and commercial production - enabling you to make the most of our innovation without ongoing financial constraints. Fill out the form to get in touch with our team of experts for more information!
Global Compliance, Local Presence
KBI Biopharma is a trusted global CDMO with a strong track record in world-class cell line development, biomanufacturing, and analytics. Our facilities in the US and Europe have been approved by the US FDA, EMA, and SwissMedic for global compliance with a local presence.

End-to-End Solution from CLD to Fill & Finish
As part of our Strategic Alliance with Argonaut
KBI Biopharma's End-to-End Offering Includes Fill Finish Capabilities as Part of our Strategic Alliance with Argonaut.
Through our alliance with Argonaut, KBI Biopharma is pleased to offer integrated drug substance and drug product solutions. This collaboration reinforces our commitment to finding innovative ways to support you in bringing therapies to market quickly and efficiently. Now you can benefit from seamless drug product manufacturing.
Argonaut
- A flexible and reliable partner with a state-of-the-art sterile facility inspected by the FDA, PMDA and EMA
- Positioned as an industry leader with a diverse customer base, ranging from small biotech to top-10 large pharma
- Demonstrated leader in the industry, capable of supporting clinical through commercial campaigns
- Commitment to quality and efficiency that aligns with KBI’s values and vision without compromising speed or product yield
Be Confident in Our Services
- Our fill finish services streamline end-to-end production and bring your products to patients in need, faster.
- Seamless integration and optimized supply chain practices shared by KBI and Argonaut are tracked by a single project management team.
- Through co-developed processes, shared commitment to quality, and continuous monitoring, rest assured that your project is in good hands.
Filling Capabilities Include
- 2R to 20R Vials Validated, 30R change parts available
- Run rate: 2,000 vials/hr x 2R vials max 10 hours
- Run rate: 1,000 vials/hr x 20R vials max 10 hours
- Batch size: 100 to 20,000 x 2R vials (10-hour fill)
- Nitrogen gas applied at two stations
- 100% weight checks
- Introduction of pre-filled syringes in 2025

*The SUREmAb offer does not apply to mAb-based biosimilar projects or for any IgG shape-derived proteins that are different from an intact IgG format including bsAbs, Fc fusions, IgG fragments, and other protein classes outside o.
Monoclonal Antibody Development, the Way it's Meant to be
KBI Biopharma is a trusted global CDMO with a strong track record offering best-in-class mammalian-based expression for breakthrough molecule types.
Talk to our Experts
© 2025 KBI Biopharma