Mammalian
Cell Line Development
CHO-based mammalian expression for robust and versatile cell line development with high productivity and performance. Matched to your molecule and needs.
Advanced Mammalian Cell Line Development for Manufacturing New Biologics
Mammalian-Based Expression Know-How to Discover, Develop, and Manufacture
Leveraging advanced gene technology and our proprietary, advanced cell line to meet the challenges of mammalian cell line development (CLD) for unique and innovative biologics.
At KBI Biopharma, as a global partner for mammalian-based biopharmaceutical development, we have engineered the standard Chinese Hamster Ovary (CHO) workhorse to create our premium SURE CHO-M Cell Line™ for mammalian-based expression.
Mammalian cells are the gold standard for biologics as a key expression system for the production of biotherapeutics. Our premium mammalian cell line development services overcome common expression bottlenecks related to transcription, translation, DNA repair, secretion, protein folding, glycosylation, and more.
At KBI Biopharma, we know that best-in-class technology is the foundation of premium mammalian cell line development. Building upon that foundation, our extensive expertise enables full-scope planning, which we use to tailor our mammalian cell line development services to your molecule and needs.
Our three types of mammalian cell line development programs range from plug-and-play rapid development solutions to tailor-made, customized programs to fit your unique molecule and mammalian cell line development needs:
- Standard new mAbs:
transfection to RCB in as little as 9 weeks with our SUREmAb™ offering - Standard variant screening:
(sequence variant or signal peptide variant), transfection to lead identification in as little as 8 weeks - Standard cell quality attributes:
(for bispecifics, biosimilars, Fc-fusion, and difficult-to-express proteins), from transfection to lead evaluation in as little as 11 weeks and from transfection to RCB in as little as 18 weeks.
A Match for Breakthrough Molecule Types
Our proprietary SURE CHO-M cell line development tools and technologies significantly reduce the time, effort, and costs associated with developing high-performance mammalian cell lines. Our SUREtechnology Platform™ has a track-record of successfully expressing a diverse range of molecules with high titers, including classical IgGs, bispecifics, Fc fusions, enzymes, hormones, blood factors, and vaccines, among others, whether they are biosimilars or new biologic entities.
- With our modular workflows, we can quickly generate CHO-M clones for expression and production of the desired recombinant protein
- Leveraging a comprehensive panel of analytical methods early in the SURE CHO-M cell line development process enables early characterization of the expressed protein
- Early characterization ensures only those cell clones producing proteins with the desired attributes associated with high viability and cell density are advanced through the SURE CHO-M cell line development process
- Our fed-batch strategies enable high representability of large-scale production in bioreactors in the earliest phases of SURE CHO-M cell line development with various volume ranges from small quantities for lead identification to toxicology studies material supply
- Our IND-ready data package accelerates IND submission while mitigating manufacturing risks thanks to our thorough genetic characterization, monoclonality assessment, and full documentation of the RCBs
Ready to Get Started?
Explore More Mammalian:
Let's Chat.
Fill out the form below to get in touch with our team of experts.
The KBI SUREtechnology Platform™, Powered by Selexis®
Advancing New Biologics to the Clinic, Faster
The SUREtechnology Platform™ creates stable, high-yield, and clonal mammalian cell lines
The SUREtechnology Platform facilitates the rapid, stable, and cost-effective production of virtually any recombinant protein and provides seamless integration of the new biologics continuum, spanning from discovery to commercialization.
By combining our proprietary SUREtechnology Platform with our world-class expertise, our mammalian cell line development services significantly streamline the development of high-performance mammalian cell lines for therapeutic protein production. As a cornerstone of the SUREtechnology Platform, the KBI SURE CHO-M Cell Line™ is a proprietary, high-performance cell line derived from CHO-K1 cells and used for the production of biotherapeutics. The SURE CHO-M Cell Line was specifically developed to overcome protein expression bottlenecks, leading to optimized timelines and exceptional versatility.
Monoclonality Assessment
Genomic Characterization for Strategic Decision-Making
Characterizing transgene-genome junctions is mandatory for IND filing and for assessing monoclonality of research cell banks (RCBs) and master cell banks (MCBs). These genomic data are critical to the success and cost-effectiveness of biomanufacturing.
Our genomic characterization of mammalian RCBs encompasses monoclonality assessment using an unbiased, blind approach to the identification and validation of integration sites. When it comes to regulatory compliance, the expectation is that cell banks are thoroughly documented for clonal derivation. Our Whole Genome Sequencing (WGS) applications deliver the data you need for mammalian cell line development, genomic characterization, and monoclonality assessment - quickly and with great accuracy. Our services and ready-to-use data packages support the decision-making process and regulatory filings.
Tailoring solutions around your needs, we leverage proprietary technologies and platforms to provide you with a fast and robust genomic characterization of your RCBs or to empower your position when facing regulatory authorities by assessing your cell line monoclonality.
Get to First in Human, Faster
Manufacturability Assessment
Determine your Lead Molecule from an Early Stage
Our manufacturability assessment package provides customers an economic tool to select their lead molecule out of a few candidates with the most stable profile at a very early stage.
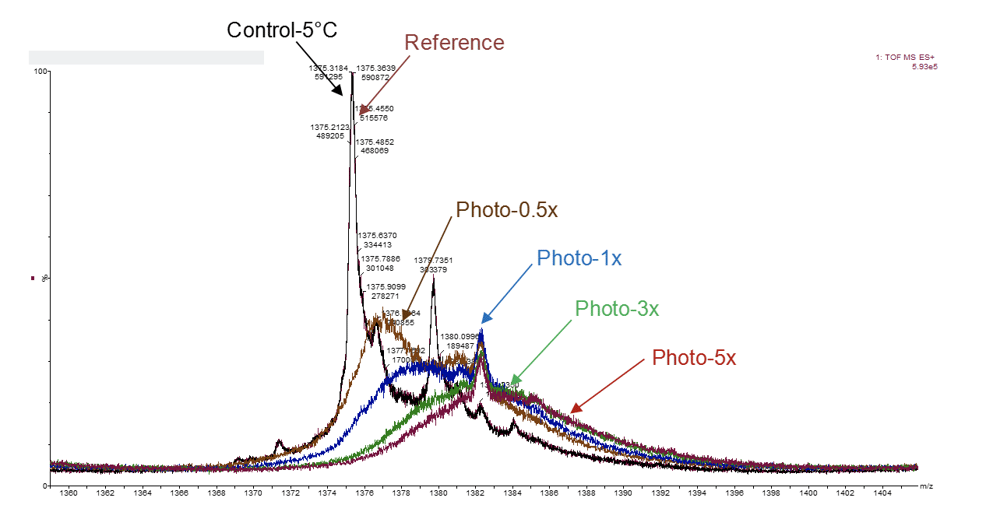
Candidate Selection
In parallel to your development within a CRO or in parallel to our CLD, we enable you to identify your lead candidate from transient or stable cell pools or intermediate clones to accelerate transition into complete CMC programs.
Advanced Analytics For CQA Assessments:
- Intact Mass Spec
- Glycan profiling
- DSF/DLS
- Accelerated stability programs
- ELISA/Octet potency assessment
- Minimum formulation screening
Transient Expression CHO Platform and Rapid Material Generation
Early candidates screening in support of your development and cost-control needs
With your R&D material needs in mind, we can quickly generate material to screen your candidate molecules for toxicology studies, reference materials, and various other R&D testing needs. It is crucial to screen and select top drug candidates early on. This strategy mitigates the risk for expensive potential roadblocks, enabling impactful cost-savings while ensuring strong product quality under accelerated timelines. As an excellent cost-effective approach, our rapid material generation CLD services are comprised of:
- Transient expression platform based on our SUREtechnology™ and SURE CHO-M Cell Line™
- Proven to reach up to 1g/L titer across 8 biomolecules formats with high purity and reliable performance trends (titer, purity, charge distribution and glycan distribution) over stable expression with the SUREtechnology Platform
- Transient expression experience in HEK293
- Rapid material generation for early discovery needs
Download our poster and see how our transient protein transfection produces biotherapeutics with high yield and purity, while also giving reliable trends in titers and product quality (PQ) profile over stable expression.
Contact Us
Fill out the form below to connect with our team of experts.
Explore More Mammalian Services & Capabilities

Never Miss Another Update!
Subscribe to KBI's Newsletter, The Pulse, to stay up-to-date on all the latest news, articles, and events from KBI Biopharma.