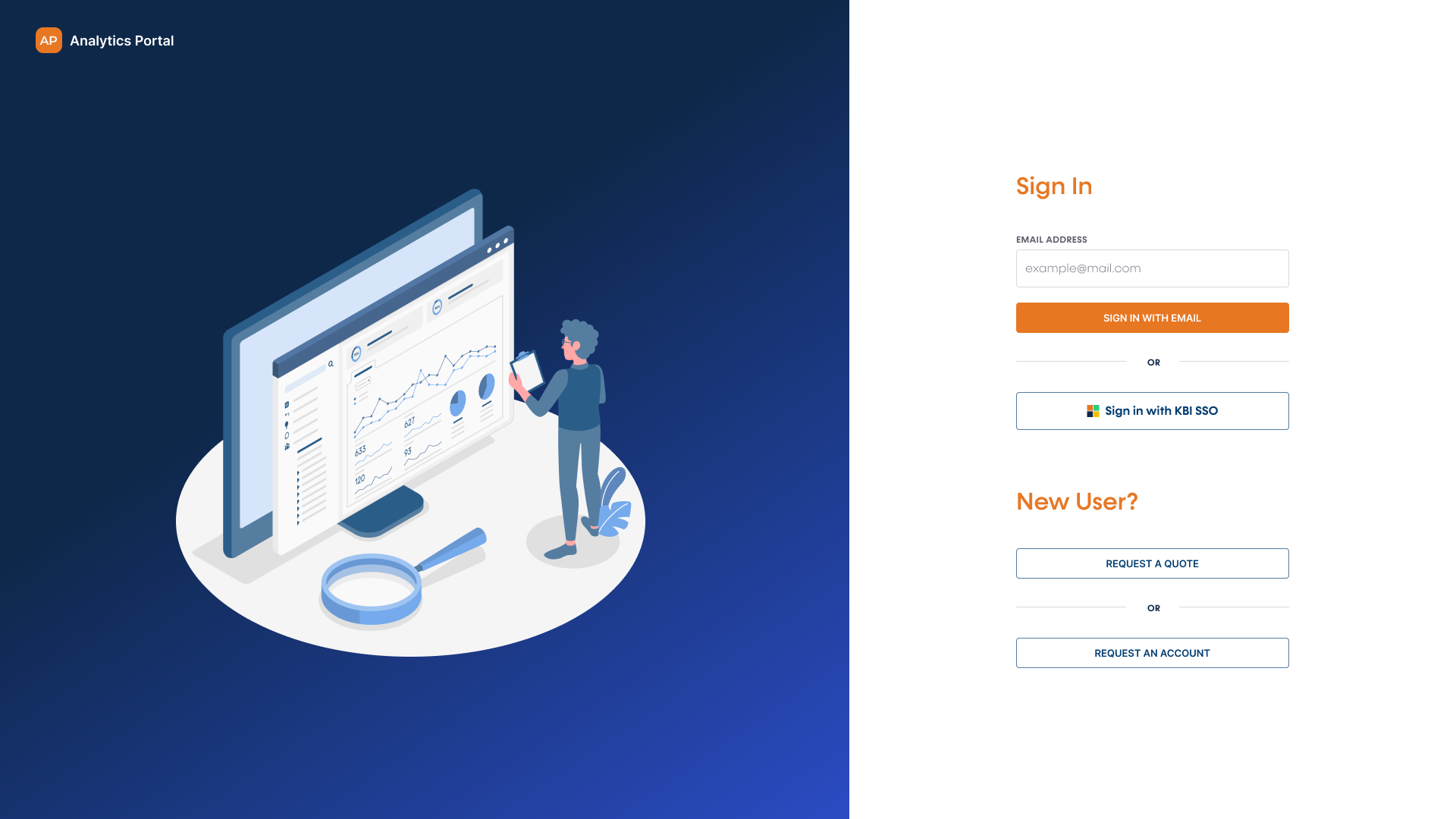
Easy Access. Timely Results. All in One Analytics Portal
Effortlessly manage your analytical testing needs from quote request to data report access and meet your analytical testing deadlines with ease
Launch the Analytics Portal
Expert Analytics To Support Robust Data Requirements
Self-Service Analytical Support at Every Phase of the Project Cycle
The KBI Biopharma Analytics Portal is a central hub for quote requests, ordering, sample submission and shipment tracking, as well as a unified visualization of studies. By building upon our digitalization, we are able to provide you with streamlined digital and live access to your Analytics program, from developability to GMP and up to Biosafety Level 2. The Analytics Portal is your go-to tool for your analytics programs.
Elevated Access & Analytics
Through a comprehensive quality assurance system, we ensure the highest quality of services at levels, from GLP to GMP. We offer analytical and formulation services from development phase, where we support your efforts with versatile and tailor-made services; all the way to and beyond commercialization, where we offer services in GMP compliance in dedicated facilities with full QA involvement.
Meeting Stringent Compliance with Data Integrity
KBI Biopharma operates all projects under stringent cGXP compliance, ensured with digital operations at every step through Electronic Lab Notebooks (ELN), Laboratory Information Management System (LIMS), and Manufacturing Execution System (MES). Digitization assures operational excellence of lab procedures is consistently performed under high industry standards with high level data integrity, traceability, and documentation. Our compliance and quality digital system secures the highest quality from sample submission all the way to the final report.
GMP-Accredited Services
KBI Biopharma has received GMP accreditation for several analytics and formulation methods. Our GMP-compliant analysis is performed using qualified, state-of-the-art instruments and meticulously developed and comprehensively validated methods. Full GMP documentation and supervision by Quality Management enable us to deliver comprehensive analytics data packages and generate supportive data for market approval.
Specialized GMP-Compliant Techniques
KBI Biopharma offers many of its services under full GMP compliance and we are constantly adding new services and analytical techniques to our GMP portfolio. Here are some prominent examples of our GMP Analytical expertise, that we offer up to BSL2:
- Binding Assays
- Characterization, including GMP Analytical UltraCentrifugation
- Chromatography
- iCEF and CE
- Mass Spectrometry
- Microbiology
- Particulates
- and more
Through a comprehensive quality assurance system, we ensure the highest quality of services at levels, from GLP to GMP. We offer analytical and formulation services from development phase, where we support your efforts with versatile and tailor-made services; all the way to and beyond commercialization, where we offer services in GMP compliance in dedicated facilities with full QA involvement.
KBI Biopharma operates all projects under stringent phase appropriate data integrity, ensured with digital operations at every step through Electronic Lab Notebooks (ELN), Laboratory Information Management System (LIMS), and Manufacturing Execution System (MES). Digitization assures operational excellence of lab procedures is consistently performed under high industry standards with high level data integrity, traceability, and documentation. Our compliance and quality digital system secures the highest quality from sample submission all the way to the final report.
At KBI Biopharma, we work with our customers to provide analytical services with phase appropriate data integrity and adherence to ICH guidelines and cGMP requirements. Our customized approach is based upon over 25 years of experience in CMC analytics from pre-clinical R&D through to market approval and beyond. Our approach is designed to provide you with the right balance of risk and cost at each stage of your CMC program to enable a successful commercial launch.
KBI Biopharma offers many of its services under full GMP compliance and we are constantly adding new services and analytical techniques to our GMP portfolio. Here are some prominent examples of our GMP Analytical expertise, that we offer up to BSL2:
- Binding Assays
- Characterization, including GMP Analytical UltraCentrifugation
- Chromatography
- iCEF and CE
- Mass Spectrometry
- Microbiology
- Particulates
- and more
Ready to Get Started?
Streamlining analytics ordering and sample coordination
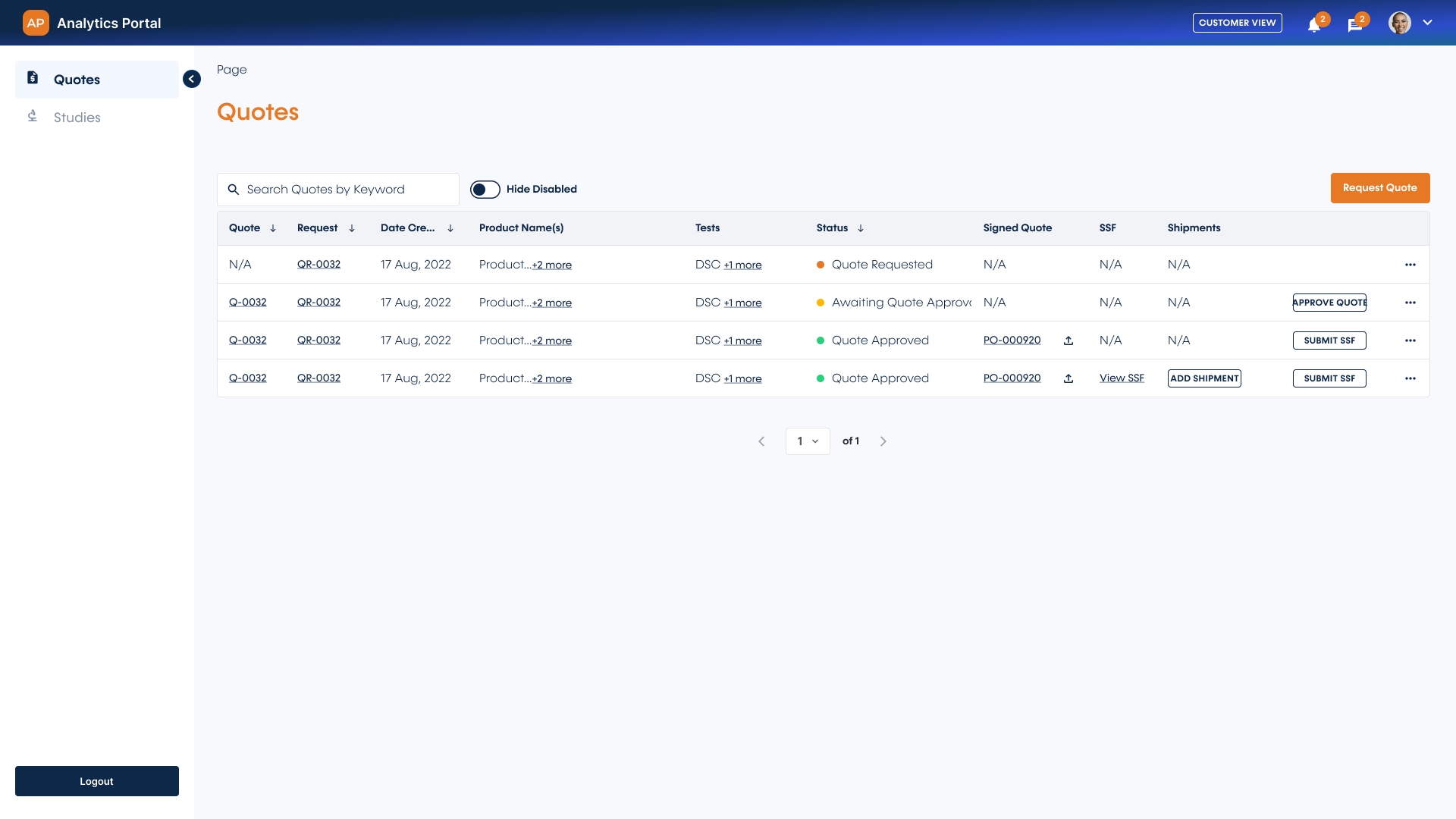
Customer-centric quote management
Request, review, and accept quotes quickly within the intuitive customer portal interface. You can easily request a quote for multiple tests and samples, ensuring the quote you receive is exactly what you need - in just a few clicks.
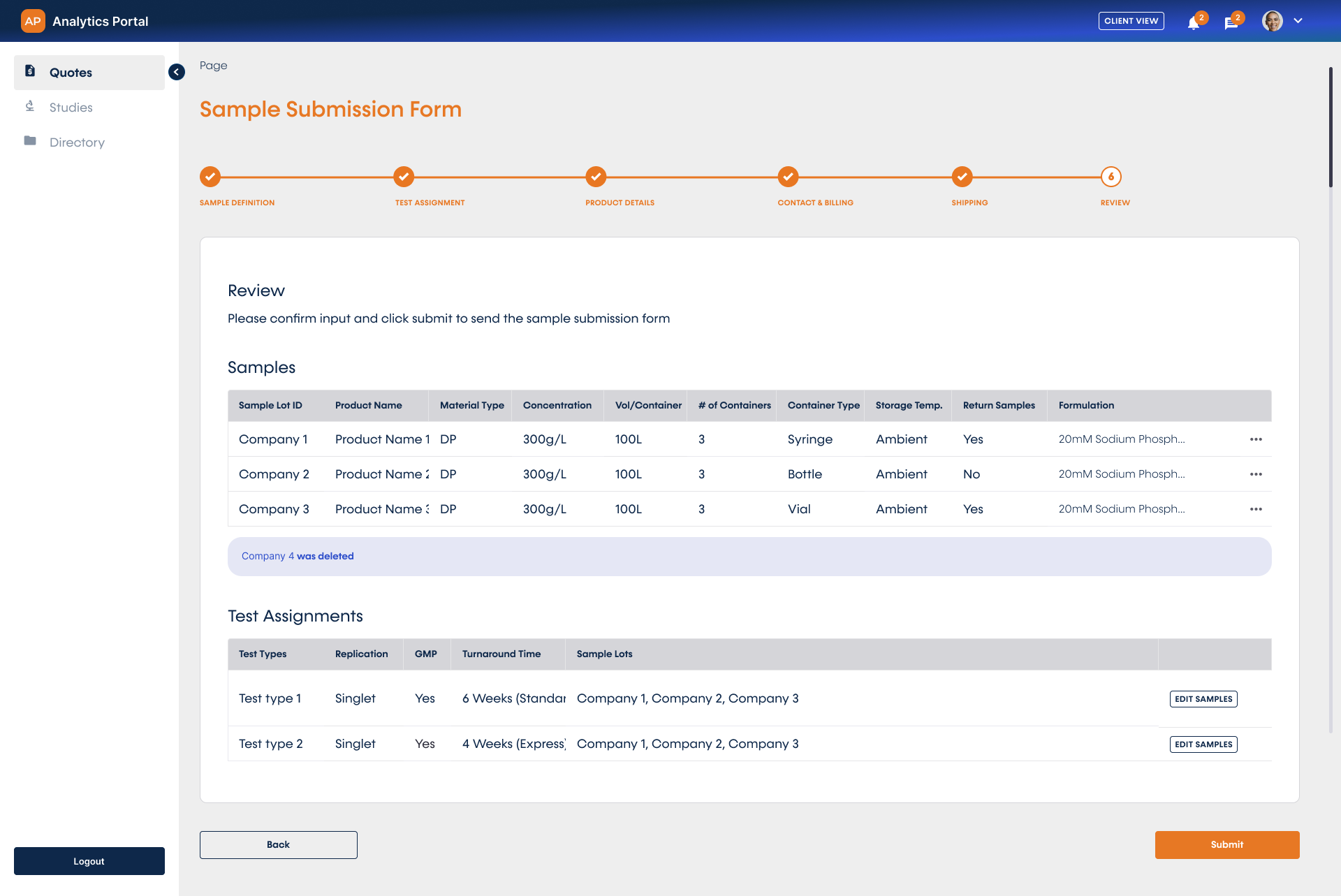
Sample Submission and Real-Time Tracking
Hassle-free sample submission can be completed directly within the portal interface. Easy shipment tracking and testing updates are now available to you in real-time.
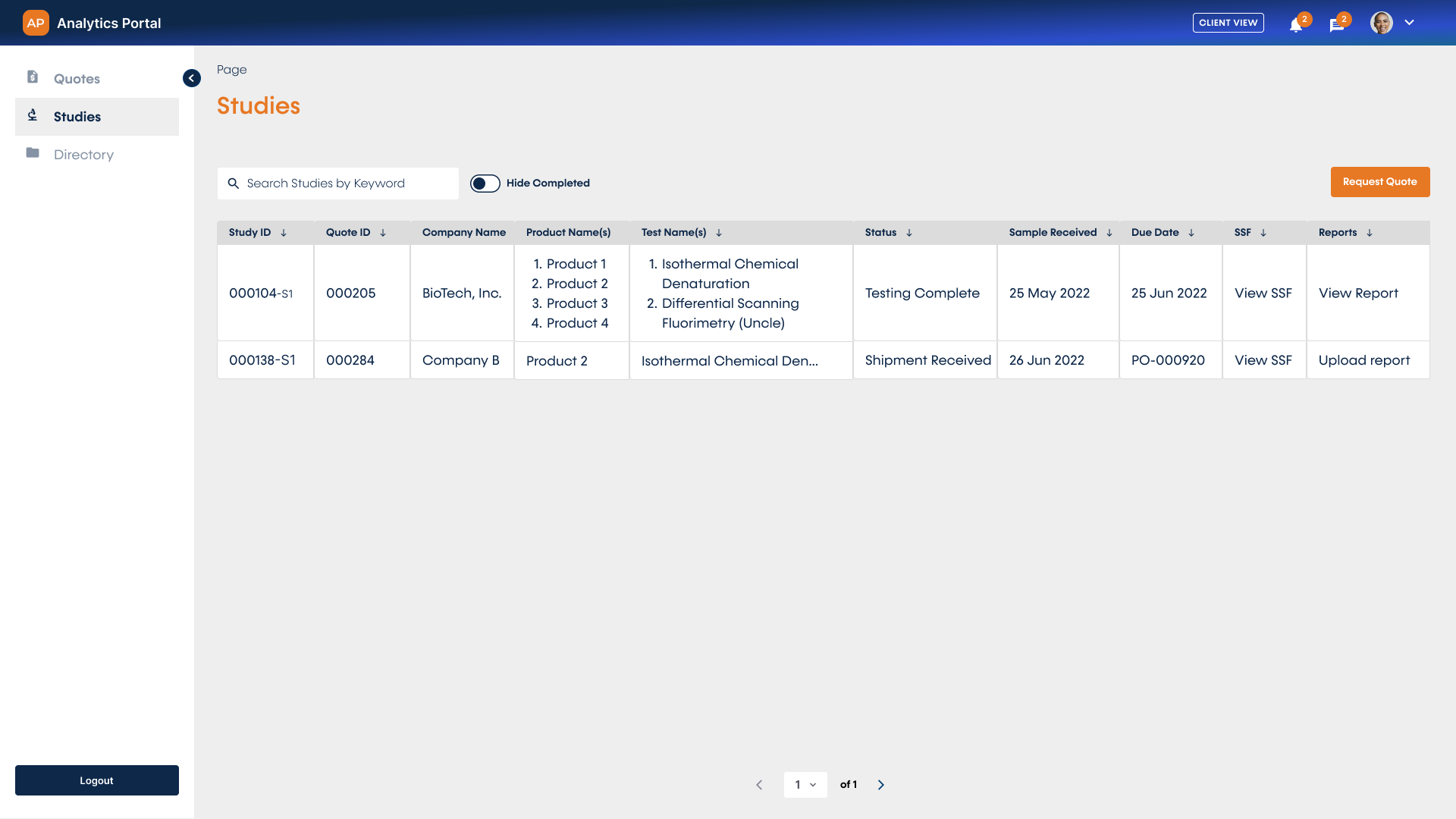
Unified Visualization of Studies
View all of your quote requests, sample submissions, and studies in a single place for a holistic understanding of your analytical projects. You can also view and download reports and results directly from the portal as they become available.
Explore More Analytical Services & Capabilities:

Never Miss Another Update!
Subscribe to KBI's Newsletter, The Pulse, to stay up-to-date on all the latest news, articles, and events from KBI Biopharma.