Mammalian
Integrated services for first in human, matched to your molecule
Engineering New Biologics, At Scale
Making Complex Applications Possible with Mammalian-Based Expression for New Biologics
With a solid foundation in analytics combined with our best-in-class cell line development (CLD) platform, KBI provides vertically integrated mammalian CDMO services for First in Human (FIH) programs from CLD through to commercial manufacturing.
The increasing complexity of novel therapeutic proteins adds pressure to established modes of expression, including Chinese Hamster Ovary (CHO) cell lines. At KBI Biopharma, as a global partner for mammalian-based biopharmaceutical development and manufacturing, we have engineered this standard workhorse to create our premium proprietary cell line for mammalian-based expression.
Mammalian cells are the gold standard for biologics as a key expression system for the production of new biologics - including therapeutic proteins like monoclonal antibodies (mAbs). CHO cells, specifically, are used for around 70% of recombinant biopharmaceutical proteins. KBI's mammalian CDMO services combine our premium cell line development services with deep expertise in analytical development, process development, and formulation development to create a comprehensive mammalian-based biopharmaceutical development and manufacturing workflow that can solidify mammalian expression for a broad variety of molecule types from CLD to infinity.
A Match for Breakthrough Molecule Types
KBI is redefining how mammalian-based expression is used throughout the development and manufacturing of new biologics, which ultimately strengthens the role of CHO cells in biopharmaceutical protein generation.
- More than 20 years of world-class innovation, pioneering exceptional cell line development
- KBI's SUREtechnology Platform™, powered by Selexis®, including our proprietary KBI SURE CHO-M Cell Line™
- Expertise in high-value bispecific and multispecific antibody production
- Robust and versatile platforms and collaborative workflows for difficult-to-express proteins and bispecific antibodies
- Cost-effective, simplified workflow for mAb development and manufacturing
Explore More Mammalian:
Let's Chat.
Fill out the form below to get in touch with our team of experts.
More Power to Develop, Manufacture, Repeat
Innovative New Biologics Come to Life with Efficient Workflows
Protein and Process Expertise, Matched to Your Molecule
Our depth of experience and expertise in mammalian-based biopharmaceutical development and manufacturing streamlines the creation of new biologics and breaks through common bottlenecks and barriers.
KBI Biopharma is a global partner for expert mammalian-based biopharmaceutical development and manufacturing. Our team of experts and world-class facilities around the globe ensure your success in developing new biologics. From cell line development to comprehensive analytical, process, and formulation development through to clinical and commercial manufacturing, working with KBI Biopharma delivers a streamlined experience.
When it comes to breakthrough molecule types, you have met your match.
KBI offers a variety of mammalian CDMO services, enabling us to provide full support for full-scope mammalian programs with the depth of knowledge that makes the complex possible. Our team of experts delivers top-tier formulation experience with backing analytics. Future-proof your program with sustained excellence for every molecule at every stage of your mammalian-based biopharmaceutical development and manufacturing journey.
Integrated Mammalian-Based Workflow for Phase I Programs
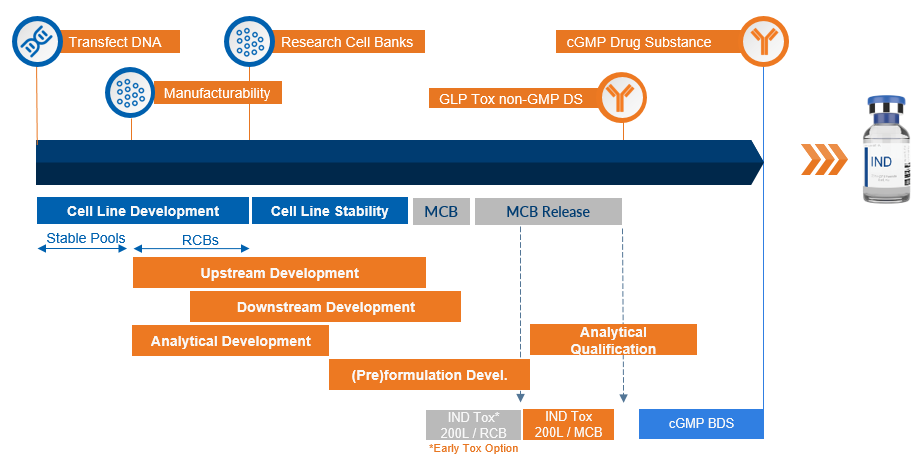
Mammalian Cell Line Development
As part of our mammalian CDMO solutions, our mammalian cell line development services leverage the SUREtechnology Platform, powered by Selexis, to provide you with best-in-class robust research cell banks (RCBs) generated from our proprietary SURE CHO-M Cell Line (CHO-M). These mammalian cell line development services include:
- Genetic engineering: transgene codon optimization and signal peptide matching with integration in proprietary vectors containing proprietary epigenetic elements optimized for the CHO-M.
- Optimized transfection protocols into the CHO-M
- Cutting edge single cell cloning technologies
- Assessment of lead RCB in ambr15 bioreactor system, including early quality check for your molecule and cell line
- Assessment of robust quality and transgene sequence verification
- Stability studies
Building upon the SUREtechnology Platform, our leading mammalian CLD services, vertically integrated as part of our CDMO integrated offering, allow us to further streamline your path to GMP with dedicated offerings matched to your molecule.

Mammalian Monoclonality Assessment
Characterizing transgene-genome junctions is mandatory for IND filing and for assessing the monoclonality of both research cell banks (RCBs) and master cell banks (MCBs). This genomic data is critical to the success and cost-effectiveness of biomanufacturing and clinical trials.
- Rapid, robust genomic characterization
- Proprietary technologies, platforms, and processes
- Ready-to-use data packages to support decision-making and regulatory filings
Using a combination of whole genome sequencing by next-generation sequencing with Illumina technology, and proprietary bioinformatics tools, we are able to develop a comprehensive data package that is ready-to-use for IND filing.
Mammalian Process Development
The clinical and commercial success of new biologics hinges on developing robust, reproducible, and scalable processes. Our process development activities cover the full development cycle:
- Early-stage discovery efforts
- Small-scale protein production
- Fully-integrated, comprehensive process development leading to GMP manufacturing
- Process characterization
- Scaled-down validation studies
Our deep knowledge and experience in the science and practice of biopharmaceutical drug development make us an ideal partner for process development.
Mammalian Analytical Services
KBI employs a phase-specific lifecycle approach to analytics. Our experience includes antibodies like IgG1, IgG4, IgM, FAb, ADC, and Fc fusion, enzymes, cytokines, growth factors, highly glycosylated proteins, protein vaccines, PEGylated proteins, conjugates, peptides, adeno-associated viruses (AAVs), oligonucleotides, and other unique proteins.
- 3000+ analytical projects
- 100+ clients
- 130+ distinct molecules
Our expertise includes HPLC, CE, ELISA, UV-Vis, mass spectrometry, light scattering, biophysical characterization (DSC, CD, FTIR, fluorescence), binding assays (ELISA, Biacore, ForteBio), glycan analyses, cell-based assays, and others.
Formulation Development
Our approach to formulation development is based on the strategic pairing of two complementary scientific disciplines: First, establishing a comprehensive understanding of the protein's thermal, physical, chemical and conformational stability, and second, employing statistical design-of-experiment (DOE) to evaluate main effects and interactions effects on protein stability. Together, these techniques enable KBI to develop robust formulations by eliminating uncontrolled stability variables, thus focusing solely on therapeutic performance and clinical outcomes.
- 130+ successful protein, peptide, and vaccine formulation development programs
- Creation of robust formulations by eliminating uncontrolled stability variables
- Stable liquid formulations for protein concentrations ranging from >1mg/mL to 200mg/mL.
KBI's data-driven approach can also strengthen responses to regulatory inquiries.
Clinical Mammalian Manufacturing
After becoming the first CMO in the world to implement the 2,000 L scale Xcellerex™ single-use bioreactor, KBI immediately began delivering GMP Drug Substance supplying first in human (FIH) and clinical resupply programs.
Our success is the result of our greatest asset - our team members. We are a diverse collection of experienced industry professionals who understand the impact these new proteins, antibodies, and vaccines have on human health. We put our hearts and experience to work, delivering outstanding quality.
Learn More: Clinical Manufacturing
Commercial Mammalian Manufacturing
Building on KBI's legacy, our commercial manufacturing suite is equipped with flexibility, taking advantage of single-use technologies for each unit operation and leveraging our extensive experience with large-scale single-use equipment.
Making Monoclonal Antibody Development a SURE thing with SUREmAb™
With optimized processes for efficiency and speed, SUREmAb delivers high titers with lower-cost workflows.


Built on the SUREtechnology Platform, SUREmAb shortens development and manufacturing for monoclonal antibodies (mAbs). It enables RCB development in as little as 9 weeks* and drug substance release in 11 months*. Specifically designed for accelerated, efficient mAb development that is robust and secured, SUREmAb projects achieve high titers of up to 10 g/L with a streamlined, lower-cost workflow that delivers the data and insights you need, the reliability and performance you expect, and the global regulatory compliance you require.
KBI Biopharma's SUREmAb offering is built on the robustness of the SUREtechnology Platform, delivering the quality you expect with a streamlined workflow for straightforward projects. Simple mAb development is a SURE thing with our rapid solution that preemptively navigates technical hurdles for the same quality under accelerated timelines.
With SUREmAb™,
you've met your Match
Efficiency and Speed, Simplified
In your mission to advance science and improve lives, every day matters. SUREmAb has been specifically designed to accelerate development timelines.
Optimized for Your Advantage
SUREmAb is engineered for robust, secured monoclonal antibody development, achieving high titers of up to 10 g/L and offering a streamlined, lower-cost workflow.
Proven Excellence You Can Trust
With over 15 years of mAb development experience, more than 150 unique therapeutic mAb projects, and 7 commercialized mAbs leveraging the SUREtechnology Platform, you're not just choosing a service - you're joining a legacy of success.
Innovation with Alleviation of Royalties
By continuing into clinical and commercial manufacturing with KBI, license fees will be waived, enabling you to make the most of our innovation without ongoing financial constraints.
* Please note: The SUREmAb offer does not apply to mAb-based biosimilar projects or for any IgG shape-derived proteins that are different from an intact IgG format - including bsAbs, Fc fusions, and IgG fragments - as well as other protein classes outside of IgGs. Timeline estimates are subject to open manufacturing capacity and may vary by project.
For additional, diverse molecule formats, our SUREtechnology Platform offers robust solutions for difficult-to-express proteins.
A Comprehensive Approach to Bispecific Antibody Production
At KBI Biopharma, we don't just focus on one aspect of bispecific antibody production - we address challenges in cell line development, production, purification, and manufacturability. Our innovative and advanced SUREtechnology Platform is designed to express a diverse range of molecules with high titers.
Learn more about our capabilities and depth of experience:
Publication:
Application of platform process development approaches to the manufacturing of Mabcalin™ bispecifics
In their article, the authors evaluate a high-yield manufacturing platform using Mabcalcin™ molecules (which consist of Anticalcin® proteins fused to an IgG), assessed to commercially-relevant scales1. This platform approach demonstrates a fast, optimized route to process confirmation that aligns with classical monoclonal antibody (mAb) approaches when it comes to timelines and overall cost.
Whitepaper:
Delivering on the Promise of Bispecifics
Given the complexity of bsAbs, a development platform for these needs three key features to be sufficiently robust: Stable and high expression of bsAbs, straightforward early screening, and a robust cell line that can handle stressors. Furthermore, advancing bsAbs into the clinic requires process development, analytical methods, and scale-up for cGMP manufacturing. Our leverageable, integrated workflow generates high-quality clinical bulk drug substances under accelerated timelines.
Article on Labiotech.eu:
Overcoming the Challenges of Bispecific Antibody Production
In a recent article published on Labiotech.eu, Séverine Fagète, Vice President of Mammalian Cell Line Development at KBI Biopharma, details the innovative and advanced approach she and her team take to overcome common obstacles and bottlenecks in bsAb development and production. KBI Biopharma's strategic approach has been applied to more than 25 bsAbs, yielding up to 99% heterodimers and paving the way for efficient production of bsAbs for clinical use.
Webinar:
Development of effective purification processes for bispecifics using affinity capture and mix-mode polishing chromatography
Get an inside look at how KBI Biopharma and JSR Life Sciences have developed processes for the purification of bsAbs using Amsphere™ A3 affinity chromatography followed by two polishing steps. Here we discuss the challenges of bispecifics purification and our approach to mixed-mode chromatography designed for bispecifics.
Publication:
Tuning the Assembly of Bispecific Antibodies by Playing on Differential Polypeptide Chain Molar Ratios
KBI Biopharma’s Mammalian Cell Line Development team recently published the results of their research in Biotechnology and Bioprocess Engineering. In their article the authors evaluate the impact of manipulating different molar ratios of three polypeptide chains, concluding that by adding gene copies at specific ratios, the expression of bsAbs can be influenced beyond the initial engineering stage.
Webinar:
Streamlined Development For Efficient Production Of Bispecific Molecules Using An Integrated Platform Process
This webinar demonstrates a breakthrough platform approach that encompasses the efficient production of bispecific molecules in an integrated, streamlined way, from CLD (cell line development) to cGMP manufacturing. This integrated process results in high-quality bulk drug substances under accelerated timelines.
Contact Us
Fill out the form below to connect with our team of experts.
Explore More Mammalian Services & Capabilities

Never Miss Another Update!
Subscribe to KBI's Newsletter, The Pulse, to stay up-to-date on all the latest news, articles, and events from KBI Biopharma.






